Breast Pump
Medical Device Class 2a
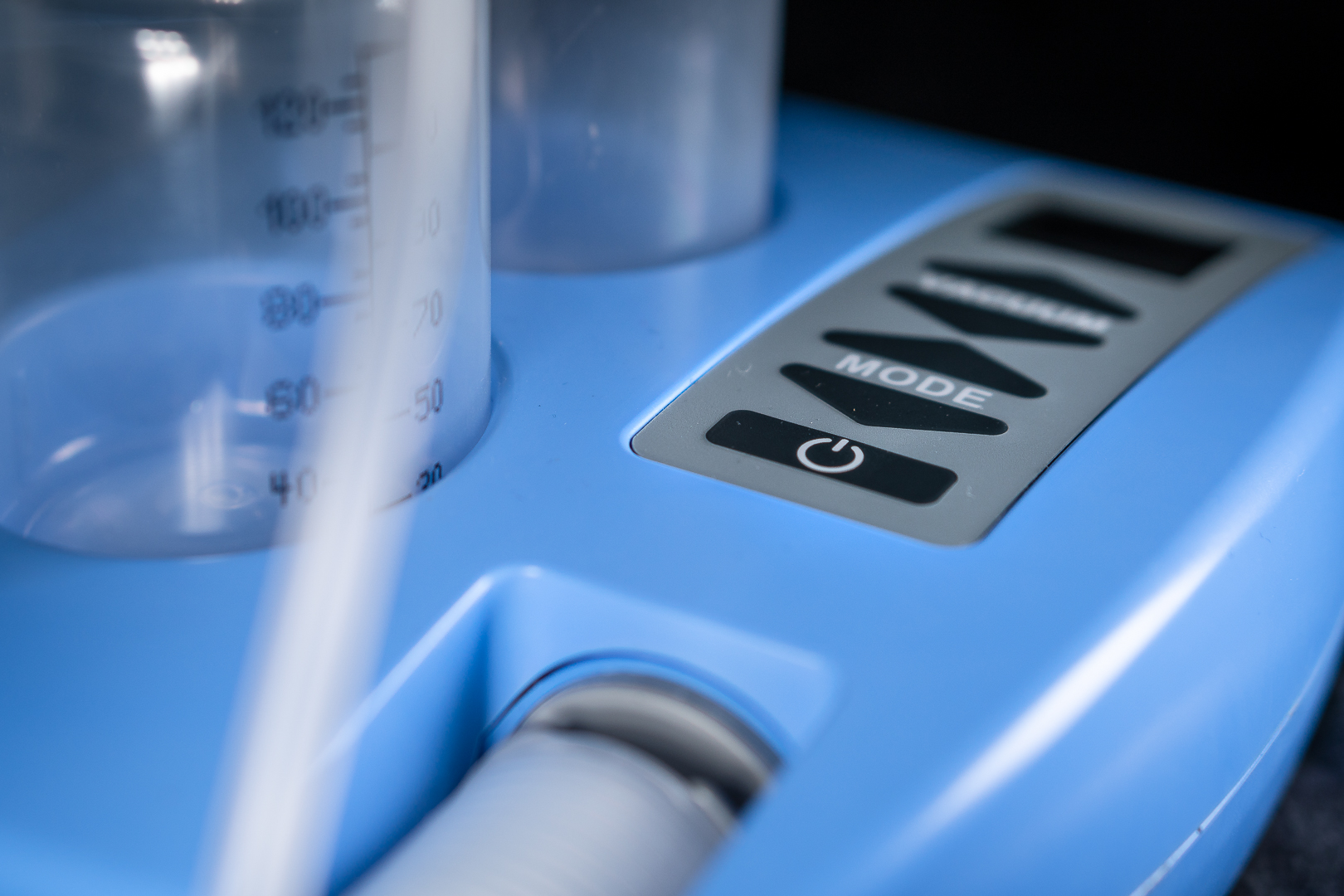
Process
The Breast Pump is a medical device. It was developed by ECT together with the client’s mechanical design engineer. Moreover, the client came with the basic idea and the pump.
ECT was in charge of:
– Hardware and firmware
– Customized components
– Implementation in ISO 13485 factory
– Regulatory tests and approvals
The breast pump is a Medical Device Class 2a. Therefore, ECT was deeply involved in the entire process of regulatory approval. This process is more extensive than for other products.
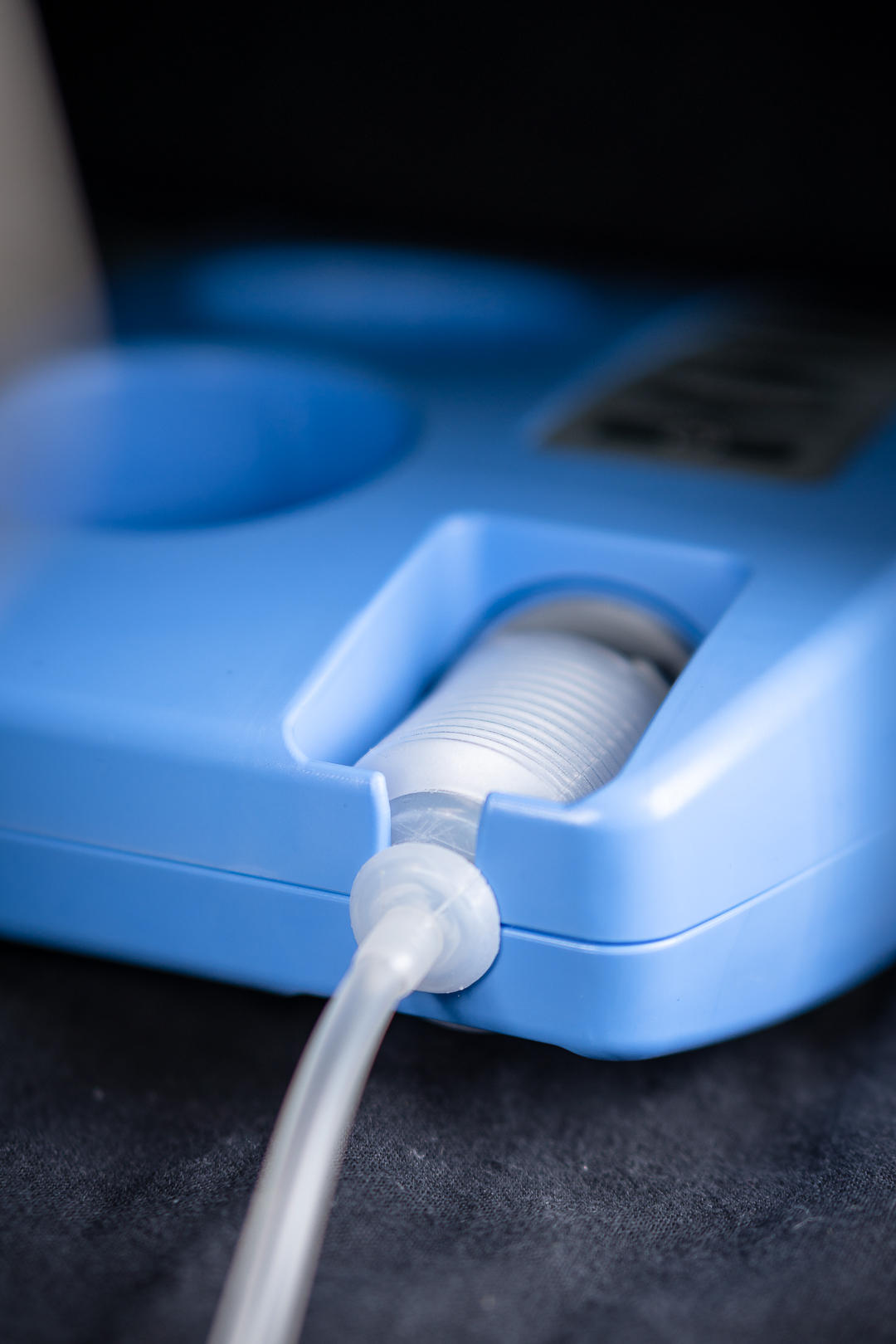
Challenge
The client wanted to create a silent breast pump with a suction pattern simulating the one of a child. Moreover, the client developed the patented, disposable pumping mechanism himself. Therefore, ECT had to develop a solution, which fitted the pumping mechanism.
Regardless whether the pump should be used in a hospital or residential, the user does not want to attract too much attention. Thus a very silent operation is important. Also an efficient and comfortable milking process was important. Thereby, ECT had to develop the product according to these demands.
Challenge
The client wanted to create a silent breast pump with a suction pattern simulating the one of a child. Moreover, the client developed the disposable pumping mechanism himself. Therefore, ECT had to develop a solution, which fitted the pumping mechanism.
Regardless whether the pump should be used in a hospital or residential, the user does not want to attract too much attention. Thus a very silent operation is important. Also an efficient and comfortable milking process was important. Thereby, ECT had to develop the product according to these demands.
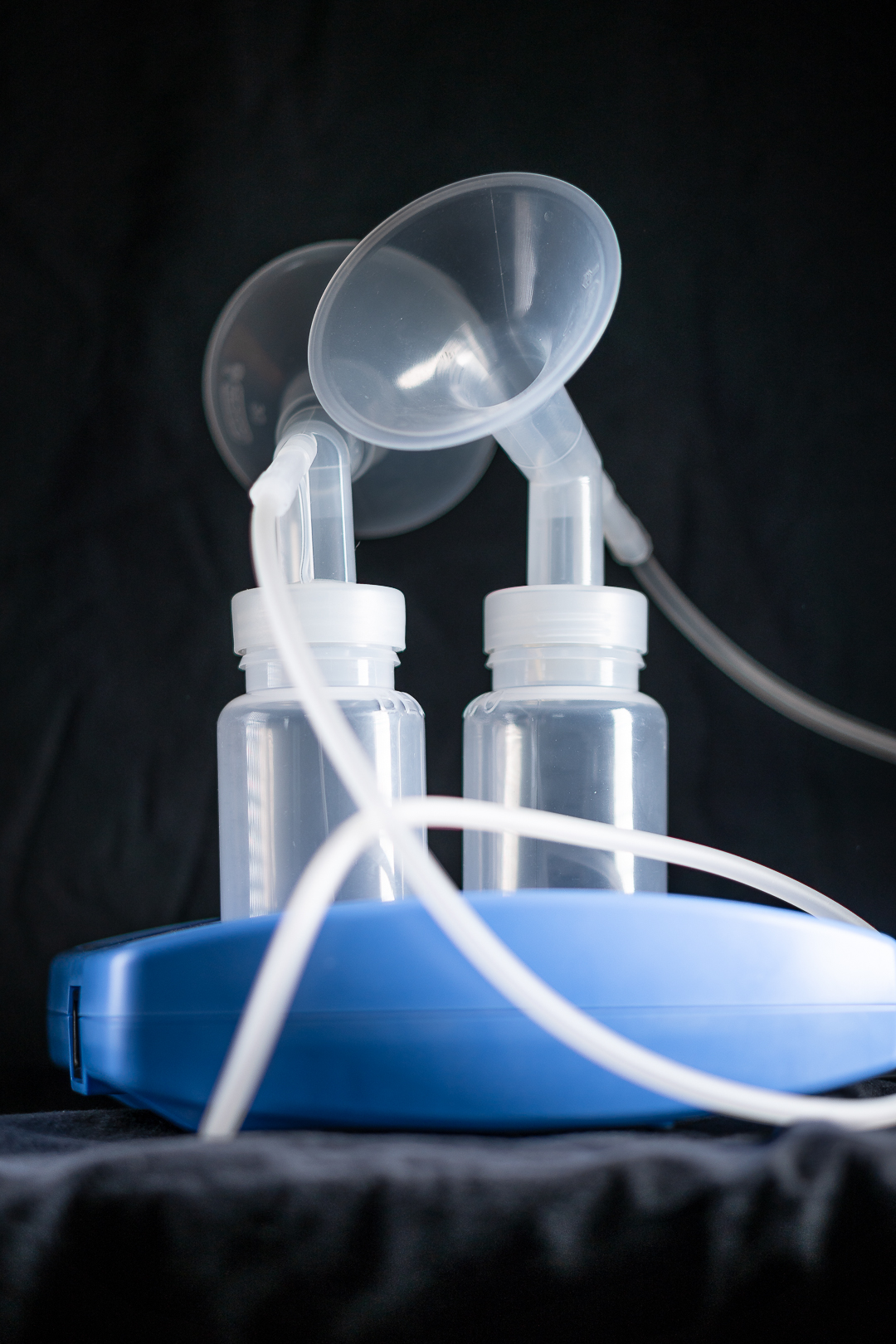
Solution
Based on inventions and patents owned by the client, the product was developed. Included in the process, we made a survey of potential suppliers for stepper motors. As a result, we decided to make a custom designed version. Thereby, it could support the claims of the product. In addition, the speed of the stepper motor was shaped to support silent pumping.
Moreover, the firmware is controlling the pumping speed and the cycle frequency. Allowing the user to select the most convenient and efficient operation in a very user-friendly way.
Also, the breast pump is classified as a Medical Class 2a device. Therefore, ECT had to participate in the clinical trials. Hereafter, the product was fine-tuned.
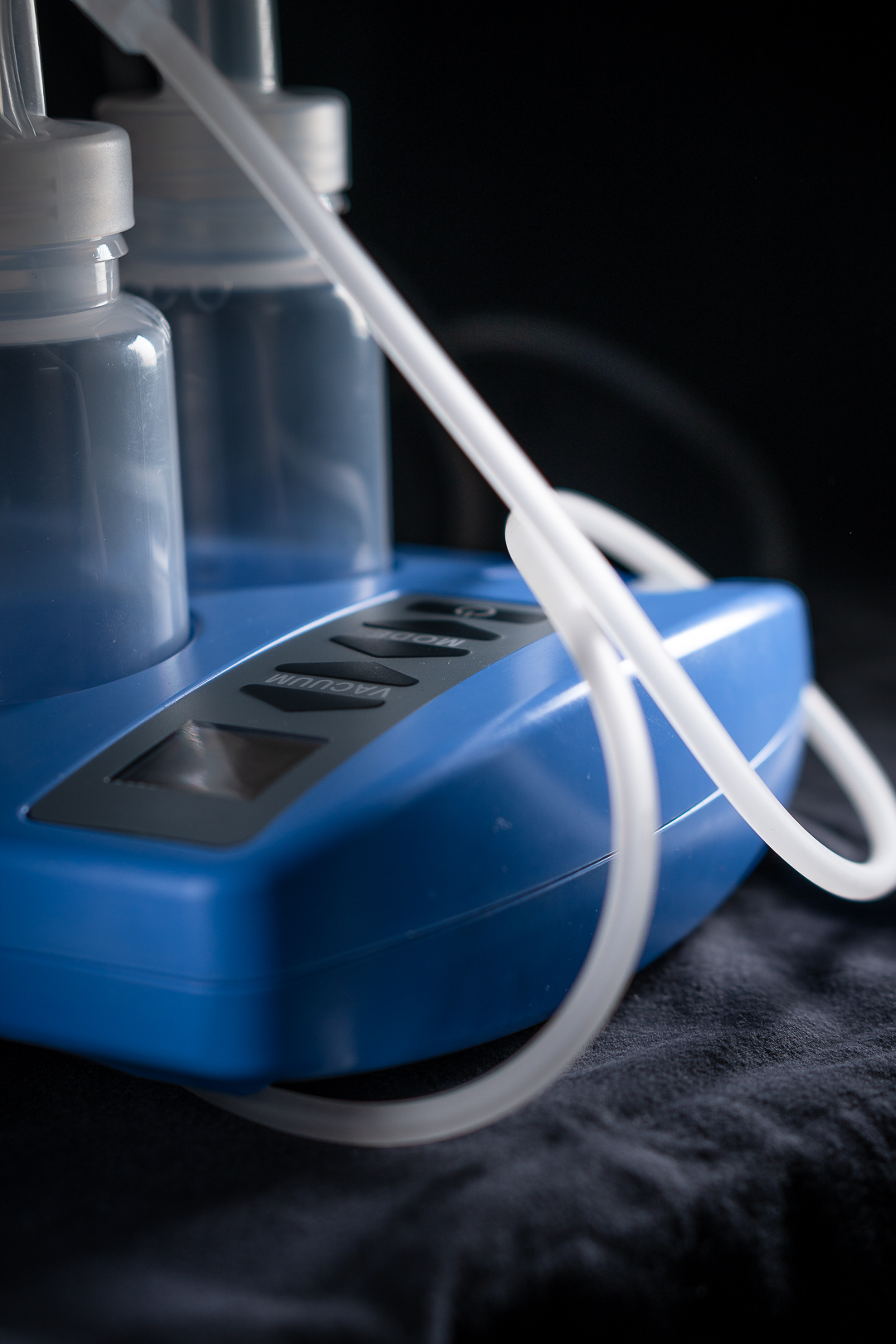
Medical Device
A good part of developing Medical Devices is related documentation. In addition, there are special rules with regards to documenting our work. Furthermore, a central document is the Risk Analysis. Here, the developers have to identify the components with the highest risk of getting defective. Including the consequence for the user hereof. In order to ensure a smooth validation process, ECT always provides all documentation in the English language.
Also, ECT implemented the breast pump in an ISO 13485 certified factory for mass production. In this process, a lot of documentation and technology information was transferred.
Medical Device
A good part of developing Medical Devices is related documentation. In addition, there are special rules with regards to documenting our work. Furthermore, a central document is the Risk Analysis. Here, the developers have to identify the components with the highest risk of getting defective. Including the consequence for the user hereof. In order to ensure a smooth validation process, ECT always provides all documentation in the English language.
Also, ECT implemented the breast pump in an ISO 13485 certified factory for mass production. In this process, a lot of documentation and technology information was transferred.








